Research
Soft condensed matter is one of the fastest growing fields of physics. It encompasses a wide range of systems, such as self-assembling nanostructures, liquid crystals, polymers and nanomaterials.
About our research
Our research combines experiments and modelling to answer questions about the structure, properties and dynamics of soft systems. It is supported by international companies, research councils and the European Union.
Our research includes both pure and applied research areas, such as crystallisation, elastomeric polymers, nanomaterials, coatings and water dynamics in porous media.
Soft matter exhibits a very rich variety of fascinating phenomena, which lead to a wide range of everyday applications, from foods to adhesives, and also to high-tech applications in optoelectronics, sensors and ‘smart’ surfaces. The group has an international profile, and research discoveries have been highlighted in world-leading journals, including Science and Nature. Particular strengths include liquid crystal elastomers, water dynamics in cement and porous media, novel nanocomposites, crystallisation, and coatings and adhesives.
We receive both public funds, from the UK's research councils and the EU, and from industry. We are part of a number of international networks including:
- RAtionalising Membrane Protein crystallisation (RAMP)
- Enginereed Calcium-Silicate-Hydrates for Applications (ERICA).
Our reputation
We have a strong reputation for research. We regularly publish our results in leading international journals, such as Physical Review Letters and Advanced Materials. In the 2014 Research Excellence Framework (REF), most of our groups research was classified as either world leading or internationally excellent.
Nanocrystal with disorder - simulation snapshot
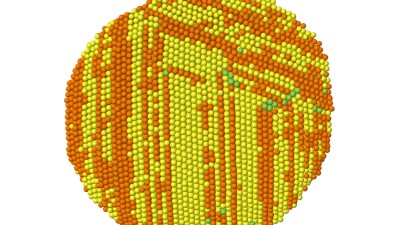
We study nanomaterials via both experiment and simulation. Shown below is a snapshot of a crystalline nanoparticle. The yellow and orange atoms are atoms in locally face-centred cubic and hexagonal close packed environments, respectively. The stripes are defects in the crystalline ordering called stacking faults. Defects in crystalline ordering affect both nanoparticle growth and their effectiveness as a catalyst.
Physics research groups
Take a look at the other research we do within the School of Mathematics and Physics.