Genomics facility
We are a shared facility, located in laboratory 19AX02, available for use by anyone within the Faculty of Health and Medical Sciences (FHMS) or Faculty of Engineering and Physical Sciences (FEPS).
Overview
We are a shared facility, located in laboratory 19AX02, available for use by anyone within the Faculty of Health and Medical Sciences (FHMS) or Faculty of Engineering and Physical Sciences (FEPS). The facility hosts equipment for the analysis and quantitation of nucleic acids and protein. All equipment is free to use, but researchers are generally required to provide their own consumables. Researchers must undergo a laboratory induction before working in our lab and a Howie-style lab coat must always be worn.
The laboratory is a clean laboratory, which means no live organisms are allowed to be brought into the lab.
Researchers are entirely responsible for the correct and proper storage of all data generated on all of the equipment in the lab. We have to regularly clean/clear data erroneously stored on the associated computers in order to keep the equipment running smoothly. Most of our computers are networked, and we strongly recommend that you back-up all your data.
Equipment
| Equipment | Purpose | Training | Booking |
|---|---|---|---|
| -80 freezer | Back-up storage facility | None available | None |
| Bioanalyzer | Measures quality of nucleic acid sample | Available on request | Online |
| Blue Pippin | Electrophoretic DNA-fragment size-selection | None available | None |
| Covaris ultrasonicator | High frequency ultrasonication | None available | None |
| Miniseq sequencer | Identification of all nucleic acid species | Mandatory | Online |
| Nanodrop | Measures quantity of nucleic acid sample | Available on request | None |
| Phosphoimager | Imaging of phosphor screens | None available | Paper diary |
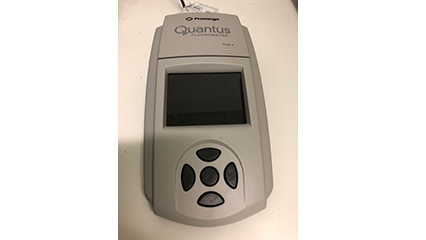
| Quantus fluorometer | Measures quantity of nucleic acid sample | Available on request | None |
| QS7 qPCR machine | Identification of specific nucleic acid species | Mandatory | Online |
| TapeStation | Measures quality of nucleic acid sample – supersedes Agilent Bioanalyser | Mandatory | Online |
| Ultracentrifuge | Ultra-speed centrifugation | Mandatory | Paper diary |
| Vilber imager | Imaging of colorimetric, fluorescent and chemiluminescent membranes/gels | Available on request | Paper diary |
Contact us
Please contact the technical team in charge of the facility for further information.
- Email: systemsbiologylabmanager@surrey.ac.uk
- Telephone: +44 (0)1483 686498
- Mobile: +44 (0)7866 459744
- Location: 33AX01
Lab team
The laboratory is supported by the Systems Biology technical team.

Dr Helen King
Laboratory Manager (shared post)

Dr Mandy Fivian-Hughes
Laboratory Manager (shared post)

James Childs Hunt
Laboratory Technician