Dr Anthony Payne
About
My research project
Computational design of sustainable 2D catalystsThis project aims at designing novel sustainable 2D materials for industrial and environmental catalysis.
This project aims at designing novel sustainable 2D materials for industrial and environmental catalysis.
Publications
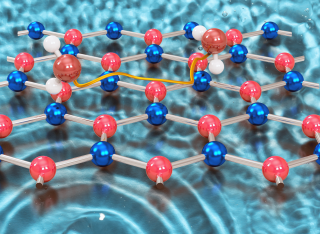
Understanding water behaviour on 2D materials is crucial for sensing, microfluidics, and tribology. While water/graphene interactions are well studied, water on hexagonal boron nitride (h-BN) remains largely unexplored. Despite structural similarity to graphene, h-BN’s slightly polar B-N bonds impart a large band gap, high thermal conductivity, and chemical stability, making it promising for electronics, lubricants, and coatings. Moreover, existing water studies often focus on multilayer water dynamics, overlooking single-molecular details. We bridge this gap by studying the friction and diffusion of individual water molecules on h-BN and contrasting it to graphene, using high-resolution helium spin-echo experiments and ab initio calculations. Our findings reveal that water diffusion on h-BN/Ni exhibits a complex, rotational-translational dynamic in contrast to its behaviour observed on graphene. While conventional views treat water motion as discrete jumps between equivalent adsorption sites, we demonstrate that on h-BN, water molecules rotate freely around their centre of mass. Although the binding energies of water on h-BN and graphene are similar, the activation energy for water dynamics on h-BN is 2.5 times lower than on graphene, implying a much lower barrier for molecular mobility. The fundamentally different diffusion characteristics which classical models cannot capture, underscores the need to rethink how we model water on polar 2D materials. Moreover, our analysis reveals that the metal substrate strongly influences water friction, with h-BN/Ni showing a markedly lower friction than graphene/Ni, in stark contrast to the free-standing materials. These findings challenge assumptions about 2D material-water interactions, highlighting the crucial role of substrate effects to understand the physical nature of interfacial water and offer insights for designing next-generation microfluidic devices that require precise water mobility control.
Large-area single-crystal monolayers of two-dimensional (2D) materials such as graphene and hexagonal boron nitride (h-BN) can be grown by chemical vapour deposition (CVD). However, the high temperatures and fast timescales at which the conversion from a gas-phase precursor to the 2D material appears, make it extremely challenging to simultaneously follow the atomic arrangements. We utilise helium atom scattering to discover and control the growth of novel 2D h-BN nanoporous phases during the CVD process. We find that prior to the formation of h-BN from the gas-phase precursor, a metastable (3 × 3) structure is formed, and that excess deposition on the resulting 2D h-BN leads to the emergence of a (3 × 4) structure. We illustrate that these nanoporous structures are produced by partial dehydrogenation and polymerisation of the borazine precursor upon adsorption. These steps are largely unexplored during the synthesis of 2D materials and we unveil the rich phases during CVD growth. Our results provide significant foundations for 2D materials engineering in CVD, by adjusting or carefully controlling the growth conditions and thus exploiting these intermediate structures for the synthesis of covalent self-assembled 2D networks.
Catalytic methane decomposition (CMD) is receiving much attention as a promising application for hydrogen production. Due to the high energy required for breaking the C-H bonds of methane, the choice of catalyst is crucial to the viability of this process. However, atomistic insights for the CMD mechanism on carbon-based materials are still limited. Here, we investigate the viability of CMD under reaction conditions on the zigzag (12-ZGNR) and armchair (AGRN) edges of graphene nanoribbons employing dispersion-corrected density functional theory (DFT). First, we investigated the desorption of H and H-2 at 1200 K on the passivated 12-ZGNR and 12-AGNR edges. The diffusion of hydrogen atom on the passivated edges is the rate determinant step for the most favourable H-2 desorption pathway, with a activation free energy of 4.17 eV and 3.45 eV on 12-ZGNR and 12-AGNR, respectively. The most favourable H-2 desorption occurs on the 12-AGNR edges with a free energy barrier of 1.56 eV, reflecting the availability of bare carbon active sites on the catalytic application. The direct dissociative chemisorption of CH4 is the preferred pathway on the non-passivated 12-ZGNR edges, with an activation free energy of 0.56 eV. We also present the reaction steps for the complete catalytic dehydrogenation of methane on 12-ZGNR and 12-AGNR edges, proposing a mechanism in which the solid carbon formed on the edges act as new active sites. The active sites on the 12-AGNR edges show more propensity to be regenerated due lower free energy barrier of 2.71 eV for the H-2 desorption from the newly grown active site. Comparison is made between the results obtained here and experimental and computational data available in the literature. We provide fundamental insights for the engineering of carbon-based catalysts for the CMD, showing that the bare carbon edges of graphene nanoribbons have performance comparable to commonly used metallic and bi-metallic catalysts for methane decomposition.
Understanding the chemical and physical mechanisms at play in 2D materials growth is critical for effective process development of methods such as chemical vapour deposition (CVD) as a toolbox for processing more complex nanostructures and 2D materials. We employ a combination of density functional theory and microkinetic modelling to comprehensively investigate the reaction mechanism governing the epitaxial growth of hexagonal boron nitride (hBN) on Ru(0001) from borazine. Our analysis encompasses four key stages prior to the formation of the complete hBN overlayer: (i) adsorption, diffusion and deprotonation of borazine, (ii) dimerisation and microkinetic modelling (iii) stability of larger borazine polymers and (iv) formation of nanoporous intermediates. In doing so, we follow for the first time the exact deprotonation sequence and illustrate its crucial role for the formation of nanostructures. Our findings do not only provide insights into the epitaxial growth of hBN and the stability of intermediate overlayers, which are strongly dependent on surface temperature and the amount of precursor exposures, they offer also crucial guidance for producing high-quality hBN monolayers with regular patterns or functionalisation. Importantly, our results align with experimental data and provide a detailed model which explains temperature-dependent, in-situ surface measurements during hBN growth on Ru.
Sustainable catalysts are essential for critical industrial and environmental processes. 2D materials have exceptional surface area and unique thermal and electronic properties, making them excellent candidates for catalytic applications. Moreover, 2D materials can be functionalised to create metalfree active sites, which provide sustainable alternatives to transition and precious metals. Among the pollutants emitted by combustion engines, NOₓ stands out as one of the most detrimental gases, contributing to environmental pollution and posing risks to human health. We demonstrate that functionalised defects in hexagonal boron nitride (hBN) provide a thermodynamically viable route to removing NOₓ by reaction with a hydrogenated boron vacancy (3HVB). The decomposition of NO₂ proceeds by initially overcoming an activation energy barrier of 1.12 eV to transfer a hydrogen atom from the surface, forming a NO₂H species, followed by the elimination of a water molecule. A thermodynamically favourable product consisting of a surface-bound hydroxyl adjacent to a nitrogen antisite defect (where a nitrogen atom occupies a site typically occupied by a boron atom) forms after overcoming an energy barrier of 1.28 eV. NO can further decompose by overcoming an activation energy barrier of 2.23 eV to form a surface HNO species. A rearrangement of the HNO species takes place with an activation energy of 1.96 eV, followed by the elimination of water. The overall reactions reduce NOₓ into defective hBN and H₂O.
This supplementary information contains the files necessary to reproduce DFT calculations contained in the publication titled "Reduction of NOx on metal-free hydrogenated hexagonalboron nitride". Please see the README_NOx for coordinate files and corresponding total energies of all structures in the article (in 'Structure_files.zip'), including geometry optimisations and transition states of the reaction mechanisms using the CASTEP program. This work made use of ARCHER2, the UK’s national high-performance computing service, via the UK’s HPC Materials Chemistry Consortium, which is funded by EPSRC (EP/R029431 and EP/X035859) and Eureka, the University of Surrey’s High-Performance Computing facility.
This supplementary information contains the files necessary to reproduce DFT calculations contained in the publication titled "Unravelling the epitaxial growth mechanism of hexagonal and nanoporous boron nitride: A first-principles microkinetic model". Please see the README for coordinate files and corresponding total energies of all structures in the article (in 'Structure_files.zip'), including geometry optimisations and transition states of the reaction mechanisms using the CASTEP program. This work made use of ARCHER2, the UK’s national high-performance computing service, via the UK’s HPC Materials Chemistry Consortium, which is funded by EPSRC (EP/R029431 and EP/X035859) and Eureka, the University of Surrey’s High-Performance Computing facility.
In this dataset, we have included computational inputs and outputs related to the paper titled “Theoretical Insights Into the Methane Catalytic Decomposition on Graphene Nanoribbon Edges”, published in Frontiers in Chemistry (doi: 10.3389/fchem.2023.1172687). The dataset comprises geometry optimizations of the catalysts employed, namely H-passivated and H-free 12-ZGNR and 12-AGNR. It also includes local minimum energy points and transition states of the reaction mechanisms, starting from the physisorption of methane and leading to the formation of solid carbon and hydrogen. Additionally, the dataset contains input/output files for molecular dynamics simulations investigating the stability of 12-AGNR and 12-ZGNR, as well as the physisorption of methane on the edges. All files are associated with the CASTEP program, with input files generally having the .in extension and output files having the .castep extension.
We report a thermodynamically feasible mechanism for producing H2 from NH3 using hBN as a catalyst. 2D catalysts have exceptional surface areas with unique thermal and electronic properties suited for catalysis. Metal-free, 2D catalysts, are highly desirable materials that can be more sustainable than the ubiquitously employed precious and transition metal-based catalysts. Here, using density functional theory (DFT) calculations, we demonstrate that metal-free hexagonal boron nitride (hBN) is a valid alternative to precious metal catalysts for producing H2 via reaction of ammonia with a boron and nitrogen divacancy (VBN). Our results show that the decomposition of ammonia proceeds on monolayer hBN with an activation energy barrier of 0.52 eV. Furthermore, the reaction of ammonia with epitaxially grown hBN on a Ru(0001) substrate was investigated, and we observed similar NH3 decomposition energy barriers (0.61 eV), but a much more facile H2 associative desorption barrier (0.69 eV vs 5.89 eV). H2 generation from the free standing monolayer would instead occur through a diffusion process with an energy barrier of 3.36 eV. A detailed analysis of the electron density and charge distribution along the reaction pathways was carried out to rationalise the substrate effects on the catalytic reaction.
This supplementary information contains the files necessary to reproduce DFT calculations contained in the publication titled "Dehydrogenation of ammonia on free-standing and epitaxial hexagonal boron nitride", published in Physical Chemistry Chemical Physics (PCCP) (DOI: 10.1039/d2cp01392d). Please see the README for coordinate files and corresponding total energies of all structures in the article including geometry optimizations and transition states of the reaction mechanisms using the CASTEP program. This work made use of ARCHER2, the UK’s national high-performance computing service, via the UK’s HPC Materials Chemistry Consortium, which is funded by EPSRC (EP/R029431) and Eureka, the University of Surrey’s High-Performance Computing facility.
A study of 16 United States Environmental Protection Agency (USEPA) priority listed PAHs associated with particulate matter < 10 mu m (PM10) was conducted in Singapore during the period 29th May 2015 to 28th May 2016. The sampling period coincided with an extensive, regional smoke haze episode (5th September to 25th October) that occurred as a result of forest and peat fires in neighboring Indonesia. Throughout this study, 54 atmospheric PM10 samples were collected in 24 h periods using a high volume sampler (HVS) and quarts fiber filters (QFF) as the collection medium. Hysplit software for computing 3D backward air mass trajectories, diagnostic ratio analysis and ring number distribution calculations were used to examine the sources of PAHs in the atmosphere in Singapore. Under normal conditions the total PAH concentrations were in a range from 0.68 ng m(-3) to 3.07 ng m(-3), while for the high haze period the results showed approximately double the concentrations with a maximum value of 5.97 ng Diagnostic ratio (DR) and principal component analysis (PCA) were conducted and indicated the contribution of the traffic as a dominant pyrogenic source of PAHs during normal periods, while results from the haze dataset showed relatively strong influence of smoke from peat and forest fires in Indonesia. Environmental and health risk from PAHs were assessed for both regular and hazy days. (C) 2017 Elsevier Ltd. All rights reserved.