Dr Ehsan Rezaee
Publications
Semiconducting molecules have been employed to passivate traps extant in the perovskite film for enhancement of perovskite solar cells (PSCs) efficiency and stability. A molecular design strategy to passivate the defects both on the surface and interior of the CH3NH3PbI3 perovskite layer, using two phthalocyanine (Pc) molecules (NP‐SC6‐ZnPc and NP‐SC6‐TiOPc) is demonstrated. The presence of lone electron pairs on S, N, and O atoms of the Pc molecular structures provides the opportunity for Lewis acid-base interactions with under‐coordinated Pb2+ sites, leading to efficient defect passivation of the perovskite layer. The tendency of both NP‐SC6‐ZnPc and NP‐SC6‐TiOPc to relax on the PbI2 terminated surface of the perovskite layer is also studied using density functional theory (DFT) calculations. The morphology of the perovskite layer is improved due to employing the Pc passivation strategy, resulting in high‐quality thin films with a dense and compact structure and lower surface roughness. Using NP‐SC6‐ZnPc and NP‐SC6‐TiOPc as passivating agents, it is observed considerably enhanced power conversion efficiencies (PCEs), from 17.67% for the PSCs based on the pristine perovskite film to 19.39% for NP‐SC6‐TiOPc passivated devices. Moreover, PSCs fabricated based on the Pc passivation method present a remarkable stability under conditions of high moisture and temperature levels.
In this report a new series of metal Schiff base complexes (MMePy, M = Pt, Pd and Cu) were synthesized and used as hole transport materials (HTMs) in perovskite solar cells (PSCs), as an attempt to realize the hole extraction and transport ability of these materials in PSCs. The energy levels of the materials can be easily tuned by changing the central metal, leading to a better alignment with the perovskite absorber. The well matching of energy level along with lower surface roughness and higher crystallinity of the thin film give rise to the highest PCE of 8.07 % for the CuMePy-based device among the MMePy-based devices. The result indicates the promising application of this series of metal complexes for the use as HTM in PSCs.
The molecular organic Lewis acid bis(pentafluorophenyl)zinc [Zn(C6F5)2] is reported as an efficient p‐type dopant for poly(3‐hexylthiophene‐2,5‐diyl) (P3HT), to be used as hole‐transporting material (HTM) in perovskite solar cells (PSCs) for the first time. To date, the most efficient PSCs use lithium bis(trifluoromethane)sulfonimide lithium salt (LiTFSI) and 4‐tert‐butylpyridine (tBP) as standard additives for HTMs. However, such dopants can induce deleterious effects on device stability. Herein, the effect of the concentration of Zn(C6F5)2 in P3HT HTM on the performance of PSCs is investigated. The P3HT‐based PSCs using a low concentration of the dopant (0.025 mol%) in the HTM layer exhibit the best performance and the highest power conversion efficiency (PCE) of 17.49%, which is almost 3.5% higher than the achieved PCE for pristine P3HT‐based PSCs. The origin of the improved performance for PSCs is further investigated, by studying the conductivity and hole mobility of the thin films based on pristine and doped P3HT. Adding a small amount of Zn(C6F5)2 to P3HT increases its thin‐film hole mobility and its hole extraction ability.
Efficient and stable hole‐transporting materials (HTMs) are necessary for perovskite solar cells (PSCs) with excellent efficiency and long‐term stability. Here, two A3B‐type metal phthalocyanine (MPc) compounds are prepared as dopant‐free HTMs for conventional n‐i‐p structured PSCs. Mono‐n‐butyl‐substituted zinc phthalocyanine and hexamethyl‐mono‐n‐butyl‐substituted zinc phthalocyanine (Me6Bu‐ZnPc) are synthesized through ring‐expansion method, and their exact structures are characterized using nuclear magnetic resonance and mass spectroscopy. The molecular orientation of the developed HTM thin films against the underlying surface is studied using X‐ray diffraction. Different substituents in MPcs can strongly affect their molecular orientation, resulting in different hole mobilities. The favored face‐on molecular alignment is only observed for Me6Bu‐ZnPc on the perovskite layer, proving the crucial role of methyl substituents in controlling the molecular alignment through the special interactions between the MPc molecule and different sites of perovskite material on the surface. PSCs using Me6Bu‐ZnPc as a dopant‐free HTM yields the highest reported power‐conversion efficiency (PCE) of 17.41%. With its high hydrophobicity and good coverage, Me6Bu‐ZnPc HTM thin film acts as an encapsulation layer, which leads to significantly increased long‐term stability. The Me6Bu‐ZnPc‐based devices retain over 90% of their initial PCE after 1400 h storage at 25 °C and with a relative humidity of 75%.
A tetra-dentate ligand L [3,3′-((pyridin-2-ylmethyl)azanediyl)dipropanamide] was synthesized and characterized by spectroscopic and structural methods. The reaction of L with two different copper(II) halides [CuX2; X = Br, Cl] in a similar condition yielded two different compounds of [LCuCl]Cl, 1 and [CuLBr]2[CuBr4]·CH3OH·H2O, 2. Both compounds were characterized by several physicochemical techniques. Single-crystal X-ray studies revealed that the Cu(II) centers in the cationic complexes 1 and 2 are in a square pyramidal N2O2X (X = Cl and Br) environment. Compound 1 is chromotropic and its solvatochromism and halochromism properties were investigated. It was found that the observed positive solvatochromism is due to structural change and solvation of the vacant site of the complex. The complex demonstrated distinct reversible spectral change over the pH range 1.3–12.1 and can act as pH-induced off–on–off absorption switch through deprotonation and the Cu–O to Cu–N bond rearrangement of the coordinated amide groups in aqueous solution.
We demonstrate a molecular design strategy to enhance the efficiency of phthalocyanine (Pc)-based hole-transporting materials (HTMs) in perovskite solar cells (PSCs). Herein, two titanyl phthalocyanine (TiOPc) derivatives are designed and applied as dopant-free HTMs in planar n-i-p-structured PSCs. The newly developed TiOPc compounds possess eight n-hexylthio groups attached to either peripheral (P-SC6-TiOPc) or nonperipheral (NP-SC6-TiOPc) positions of the Pc ring. Utilizing these dopant-free HTMs in PSCs with a mixed cation perovskite as the light-absorbing material and tin oxide (SnO2) as the electron-transporting material (ETM) results in a considerably enhanced efficiency for NP-SC6-TiOPc-based devices compared to PSCs using P-SC6-TiOPc. Hence, all of the photovoltaic parameters, including power conversion efficiency (PCE), fill factor, open-circuit voltage, and short-circuit current density, are remarkably improved from 5.33 ± 1.01%, 33.34 ± 3.45%, 0.92 ± 0.18 V, and 17.33 ± 2.08 mA cm–2 to 15.83 ± 0.44%, 69.03 ± 1.59%, 1.05 ± 0.01 V, and 21.80 ± 0.36 mA cm–2, respectively, when using the nonperipheral-substituted TiOPc derivative as the HTM in a PSC. Experimental and computational analysis suggests more compact molecular packing for NP-SC6-TiOPc than P-SC6-TiOPc in the solid state due to stronger π–π interactions, leading to thin films with better quality and higher performance in hole extraction and transportation. PSCs with NP-SC6-TiOPc also offer much higher long-term stability than P-SC6-TiOPc-based devices under ambient conditions with a relative humidity of 75%.
Herein, the important role of the isomer purity of hole‐transporting materials (HTMs) in achieving high‐performance perovskite solar cells (PSCs) is highlighted. The isomer‐pure 2,9,16,24‐tetra‐n‐butyl‐Zn(II) phthalocyanine (RE‐ZnBu4Pc) is directly synthesized through a ring expansion method, without any further purification. The ground‐state absorption, fluorescence and thermal properties of RE‐ZnBu4Pc and the isomer mixture ZnBu4Pc, along with their hole mobilities and film morphologies are investigated, proving that RE‐ZnBu4Pc can be the more efficient HTM. The devices based on RE‐ZnBu4Pc, as dopant‐free HTMs, achieve a higher average power conversion efficiency (PCE of 11.49% ± 0.67%) and more stability at 25 °C and under 75% relative humidity than that of isomer mixture ZnBu4Pc (PCE of 9.51% ± 1.15%). RE‐ZnBu4Pc‐based PSCs also show better reproducibility in the fabrication process. This study demonstrates that better device performance can be expected for PSCs with isomer‐pure HTM materials.
In this study, a nanocomposite based on graphene oxide (GO) and non-peripheral octamethyl-substituted copper(II) phthalocyanine (N-CuMe2Pc) nanorod as photosensitizer, was synthesized and characterized by X-ray diffraction, transmission electron microscopy (TEM), scanning electron microscopy (SEM), UV–vis diffuse reflectance spectroscopy, FTIR and Raman spectroscopy. For the first time, the photocatalytic activity of GO/Pc nanocomposite was tested for the reduction of aqueous chromium (VI) (10 mg/L) as a model pollutant, and the results were compared with those of pure N-CuMe2Pc and GO. Visible light was used as source and Cr(VI) concentration was monitored during 120 min of treatment period. Up to 95% photocatalytic reduction of toxic Cr(VI) ions confirms that GO/Pc nanocomposite photocatalyst is clearly effective for wastewater treatment. Meanwhile, neat GO and N-CuMe2Pc nanorod were able to reduce only less than 10% of initial Cr(VI) ions. The reusability of the developed photocatalyst was also investigated. The retained amount of Cr(VI) increased from 2% at first cycle to around 17% at third cycle, due to loss of photocatalyst during collection process and morphology changes from nanorods to nanoparticles. Our results demonstrate the remarkably improved photocatalytic performance of GO/Pc nanocomposite for the practical application in wastewater remediation.
A power conversion efficiency (PCE) as high as 19.7% is achieved using a novel, low‐cost, dopant‐free hole transport material (HTM) in mixed‐ion solution‐processed perovskite solar cells (PSCs). Following a rational molecular design strategy, arylamine‐substituted copper(II) phthalocyanine (CuPc) derivatives are selected as HTMs, reaching the highest PCE ever reported for PSCs employing dopant‐free HTMs. The intrinsic thermal and chemical properties of dopant‐free CuPcs result in PSCs with a long‐term stability outperforming that of the benchmark doped 2,2′,7,7′‐Tetrakis‐(N,N‐di‐p‐methoxyphenylamine)‐9,9′‐Spirobifluorene (Spiro‐OMeTAD)‐based devices. The combination of molecular modeling, synthesis, and full experimental characterization sheds light on the nanostructure and molecular aggregation of arylamine‐substituted CuPc compounds, providing a link between molecular structure and device properties. These results reveal the potential of engineering CuPc derivatives as dopant‐free HTMs to fabricate cost‐effective and highly efficient PSCs with long‐term stability, and pave the way to their commercial‐scale manufacturing. More generally, this case demonstrates how an integrated approach based on rational design and computational modeling can guide and anticipate the synthesis of new classes of materials to achieve specific functions in complex device structures.
New efficient hole‐transport material (HTM) composites based on low‐cost easy‐preparation non‐peripheral octamethyl‐substituted copper (II) phthalocyanine (N‐CuMe2Pc) nanowire and poly(3‐hexylthiophene) (P3HT) are developed for CH3NH3PbI3 (MAPbI3)‐based perovskite solar cells (PSCs). Compared with pristine P3HT, the prepared nanocomposite HTMs provided thin films with better qualities and reduced trap densities, and exhibited higher hole mobilities and well‐matched energy levels with the perovskite layer. Depending on the ratio of the two components, the power conversion efficiency (PCE) reached up to 16.61%, which is higher than the efficiency of the standard device based on doped spiro‐OMeTAD (16.13%). Moreover, the long‐term stability of the PSCs is also improving greatly. The best performing devices based on P1C1 HTM retained 90% of their initial efficiencies after 800 h of storage with a relative humidity of 75%. These results indicate N‐CuMe2Pc nanowire/P3HT nanocomposites can be an effective HTM to realize superior performance in PSCs.
This article reviews various dopant‐free hole transporting materials (HTMs) used in perovskite solar cells (PSCs) in three main categories including inorganic, polymeric, and small molecule HTMs. PSCs have undergone rapid progress, achieving power conversion efficiencies (PCEs) above 22%. With their low production cost and high efficiencies, PSCs are considered promising next‐generation solar cell technology. In all developed architectures for PSCs, including planar and mesoscopic with conventional and inverted structures, HTMs play a significant role in determining the photovoltaic performance of PSCs. Using p‐type dopants, however, is considered a common strategy to increase the hole conductivity of HTM, which is usually compensated by a more complicated fabrication procedure, higher production costs, and lower stability of PSC. Although several reviews on HTMs have been published, progress on dopant free HTMs needs to be reviewed and analyzed. Here, a review covering most of the published reports on dopant‐free HTMs is presented, and the device structure and fabrication method, HTM layer deposition techniques, and the efficiency and the stability of PSCs are addressed during discussions in each main category. Finally, an outlook on stability and PCE growth in PSCs based on dopant‐free HTMs is presented.
SnO2 was recently employed as an efficient electron‐transport layer (ETL) in perovskite solar cells (PSCs) and high power conversion efficiencies (PCEs) have been reported. However, it is still challenging to fabricate SnO2 thin films through facile solution‐based synthesis at low temperature (<150 °C) to be compatible with the large scale module fabrication, especially for flexible devices. Here, we report a low temperature solution‐based method for preparation of SnO2 nanoparticles. Ultrasonic‐assisted wet chemistry synthesis of ultrafine SnO2 nanocrystals with particle size ranging from 2 to 5 nm was achieved by employing a SnCl4⋅5 H2O solution in a mixed ethanol–water solution and with no annealing step. The crystallinity and microstructure of the SnO2 nanoparticles were investigated by X‐ray diffraction (XRD) and transmission electron microscopy (TEM), as well as selected area electron diffraction (SAED) analysis. The added water in ethanol and increased pH values were demonstrated as two key factors to successful fabrication of highly crystallized samples with high reproducability. An efficiency of 16.56 % was achieved for PSCs based on SnO2 nanoparticles synthesized by ultrasonic‐assisted wet chemistry.
Boron subphthalocyanine (SubPc) has special physical and chemical properties, originating from its non-centrosymmetric, near-planar taper structure and large conjugated system; it can act as an alternative to the small molecule hole-transporting material 2,2′,7,7′-tetrakis-(N,N-di-p-methoxyphenylamine)-9,9′-spirobifluorene in perovskite solar cells (PSCs). To achieve a higher solubility in common organic solvents and a more suitable highest occupied molecular orbital energy level that aligns with the valence band of the perovskite material, a SubPc molecule with a hexamethyl substitution at its peripheral position (Me6-SubPc) was successfully designed and synthesized in a one-step method. Completely solution processed PSCs were fabricated with only a small hysteresis, a power conversion efficiency of 6.96% and Voc of 0.986 V.
Solution‐processed hole transporting materials (HTMs) that are dopant‐free show promise for use in low‐cost, high‐performance perovskite solar cells (PSCs). In this study, a new HTM of copper (II) phthalocyanine with tetra‐propyl‐substituted function groups (CuPrPc) is reported. It is found that CuPrPc could form face‐on molecular orientation when spin‐coated on perovskite, resulting in high hole mobility and hydrophobic surface. These properties are more favorable for hole transport and moisture resistance applications in PSCs. Solution‐processed planar PSCs utilizing CuPrPc as HTM are fabricated and tested. PSC with CuPrPc exhibited highest power conversion efficiency of 17.8%. Furthermore, beneficial from the hydrophobic nature of CuPrPc, the devices with CuPrPc as HTM show improved stability and retain over 94% of their initial efficiency even after storage in humidity about 75% for 800 h without encapsulation, which is much better than the performance of PSCs based on Spiro‐OMeTAD HTM.
Two dopant-free hole transporting materials (HTMs) comprising a planar indacenodithiophene (IDT) core with different alkyl chains (either C4 or C6) and electron-rich methoxytriphenylamine (TPA) side arms were synthesized (namely IDTC4-TPA and IDTC6-TPA, respectively) and successfully employed in CH3NH3PbI3 perovskite solar cells. These HTMs can be obtained from relatively cheap starting materials by adopting a facile preparation procedure that does not use expensive and complicated purification techniques. In the crystal lattice, each of these molecules interacted with either the CH/π or CH/O hydrogen bonds. At the same time, the IDTC6 backbone enables a tight molecular arrangement based on π–π stacking interactions (3.399 Å); this causes the new material to have a higher hole mobility than the standard IDTC4-based HTM. Moreover, the photoluminescence quenching in a perovskite/HTM film structure was shown to be more effective at the perovskite/IDTC6-TPA interface than at the perovskite/IDTC4-TPA interface. Consequently, devices fabricated using IDTC6-TPA show superior photovoltaic properties (exhibiting an optimal performance of 15.43%) compared with IDTC4-TPA-containing devices.
The synthesis, structure, and solvatochromism properties of two new complexes with empirical formula [Cu2L2]X2, are described, where HL stands for 2-(N,N-diisopropyl-2-aminoethyl)imino-3-butanone oximato and X is perchlorate or tetraphenylborate anion. The complexes were characterized by elemental analyses, molar conductance and spectral studies. The binuclear complex [Cu2L2](BPh4)2 was analyzed by X-ray crystallography. In complexes the copper centers are bridged through the oximato NO groups forming a dimer containing a crystallographic twofold symmetry axis. The complexes are solvatochromic and their solvatochromism were investigated by visible spectroscopy. The solvent-dependent absorption maxima, λmax, was analyzed using stepwise multiple linear regression (SMLR) method to find the best model explaining the observed positive solvatochromism. The analysis demonstrated that among different solvent parameters, donor number (DN) is a dominant factor responsible for the shift in the d-d absorption band of the complexes to the higher wavelength with increasing its values.
The mixed-chelate copper(II) complexes [Cu(Ph-acac)(diamine)]X, where Ph-acac is 1-phenyl-1,3-butanedione, diamine is N,N-dimethyl,N′-benzyl-1,2-diaminoethane and X = ClO4−, PF6− and BF4−) have been synthesized and characterized spectroscopically (by IR and UV–Vis) and structurally (by single-crystal X-ray diffraction). The X-ray structure of [Cu(Ph-acac)(diamine)]ClO4 demonstrated that the central copper atom is placed in a square planar geometry made by Ph-acac and diamine chelates. Solvatochromism behavior of the compounds was studied in 14 organic solvents, which showed a positive trend with increasing donor power of the solvents. Multi-parametric equation has been utilized to explain the solvent effect on the d–d transition of the complexes using SPSS/PC software. The stepwise multiple linear regression (SMLR) method demonstrated that the donor power of the solvent plays the most important role in the solvatochromism of the compounds. To study the electronic structure of the complex and to confirm the mechanism of the observed solvatochromism, a computation analysis was accomplished on [Cu(Ph-acac)(diamine)]+ by the density functional theory (DFT) and time-dependent DFT calculations. DFT computational results buttressed the experimental observations and indicated that the solvatochromism phenomenon is due to coordination of the solvent molecules on the above and below of the molecular plane, which causes change in geometry of the complex from square planer to octahedron. In this process the d–d transition band of the complex moves to the red with increasing the DN of the solvent.
Presented here is an investigation on the geometric (molecular) structures, spectroscopic properties and electronic structures of copper(II)-nitrite complexes as a function of steric effects, utilizing a set of closely related co-ligands. The prepared complexes, with the general formula [CuLR(η2-ONO)2] where LR is N,N-dialkyl,N′-benzyl-ethylenediamine and R is methyl, ethyl or isopropyl moieties, were structurally characterized by physico-chemical and spectroscopic methods. An X-ray diffraction study on CuLMe(η2-ONO)2] and CuLEt(η2-ONO)2] reveals that the copper(II) center in both compounds is located in a distorted octahedral environment through coordination of two amine nitrogen atoms and four oxygen atoms of the nitrite ligands. Depending on the steric crowding of the co-ligand, the coordination mode is either symmetric or asymmetric bidentate, as is evident from X-ray crystallography and IR spectroscopy. The relative stability of linkage isomers of these compounds, along with a tert-butyl derivative complex, was investigated using density functional theory (DFT) calculations. The calculated results demonstrated that in all cases the bidentate η2-ONO isomer is more stable than the four-coordinated nitro isomer (η1-NO2). However, the differences in the relative stability decrease as the steric hindrance in the co-ligand increases. The vibrational spectra of the complexes were assigned using DFT calculations. In support with the X-ray structure, the results reveal that νas(N–O) splits into two bands, with the separation increasing from [CuLMe(η2-ONO)2] through to [CuLtert-buty(η2-ONO)2]. That is at least partially dictated by steric factors within the molecules, imposed by the alkyl groups of the co-ligand. The UV–Vis absorption spectra are presented and analyzed with the help of time-dependent density functional theory (TD-DFT) calculations.
A new coordination compound, [Cu(L)2(OH2)](ClO4)2 where L = N-(pyridin-2-ylmethyl)propane-2-amine, was prepared and characterized by elemental analysis, molar conductance and IR and UV–Vis spectroscopic techniques. X-ray crystal analysis of the complex confirmed that the copper(II) ion has a distorted square pyramidal environment. The complex is chromotropic in solution. The chromotropic properties of the complex, including solvato-, thermo-, halo- and ionochromism, were investigated in detail. The complex displayed strongly pronounced reversible thermochromism in solution due to dissociation and of re-coordination of a water molecule.
The present study, introduces the beneficial catalyze effects of dinuclear copper(II) complexes on the luminol chemiluminescence (CL) reaction. Two new dinuclear copper(II) complexes ([Cu2(L)2(TAE)]X2 and [Cu2(L′)2(TAE)]X2), where TAE = tetraacetylethane; L = N,N′-dibenzyl ethylenediamine and L′ = N,N-dimethyl-N′-benzylethylenediamine; X = ClO4, have exhibited highly efficient catalytic activity of luminol CL as an artificial peroxidase model at pH as low as 7.5 in water in the presence of H2O2 and dissolved O2 even in the absence of H2O2 at elevated pH (∼12). The effects of the reactant concentrations and some amino acids on luminol CL were also investigated. Among them, l-cysteine (CySH) containing -SH group was observed to inhibit the CL signal of the luminol-H2O2-dinuclear copper(II) complex. A similar phenomenon also was observed for glutathione (GSH), which made CL probe of peroxidase-like dinuclear copper(II) complexes applicable for the determination of such compounds in biological media.
Two new symmetric dinuclear complexes type [Cu(L)(μ-OH)]2(ClO4)2, where either L = N,N-dimethyl,N′-3-propylamide-ethylenediamine or N,N-diethyl,N′-3-propylamide-ethylenediamine were synthesized and characterized on the basis of elemental analysis, conductance measurement, spectroscopic techniques and X-ray crystal analysis. The complexes show halochromism in aqueous solution. The pH effects on the visible absorption spectra of the two complexes were studied which act as pH-induced “off–on–off” switches through protonation and deprotonation in aqueous solution at room temperature. The dinuclear complexes were also observed to exhibit thermochromism, solvatochromism and ionochromism as a result of hemilability of amide groups. The solvatochromism of the complexes was investigated with different solvent parameter models using stepwise multiple linear regression method. The results suggested that the donor number parameter of the solvent has a dominant contribution to the shift of the d–d absorption band of the complexes.
The catalyzed luminol chemiluminescent reaction has received a great amount of attention because of its high sensitivity and low background signal which make the reaction an attractive analytical chemistry tool. The present study, introduces the beneficial catalytic effects of dinuclear Cu(II) complex [Cu2L2(TAE)]X2, where TAE = tetraacetylethane; L = N,N’-dibenzylethylenediamine and X = ClO4 on the luminol chemiluminescent reaction as a novel probe for the determination of glutathione (GSH) and L-cysteine (CySH) in human serum and urine. The [Cu2L2(TAE)]X2 has exhibited highly efficient catalytic activity of luminol CL as an artificial peroxidase model at pH as low as 7.5 in water in the presence of H2O2⋅GSH and CySH can induce a sharp decrease in CL intensity from the [Cu2L2(TAE)]X2-catalyzed luminol system. Under the selected experimental conditions, a linear relationship was obtained between the CL intensity and the concentrations of GSH and CySH in the range of 1.0 × 10−7–1.0 × 10−4 M, with detection limits (S/N = 3) of 2.7 × 10−8 and 6.8 × 10−8 M and RSD < 4.2% (n = 7) for GSH and CySH, respectively.
This article presents a combined experimental and computational investigation of 3-amino-1-phenyl-2-buten-1-onato, APBO ligand and its copper(II) and nickel(II) complexes. APBO is an unsymmetrical, bidentate and monoanionic ligand with different coordinating atoms (N,O). A comparison among different possible conformers of the ligand has been carried out using density functional theory (DFT) method at the B3LYP/6-31+G(d,p) level. It was demonstrated that two factors control stability of the compounds as hydrogen bonding (conventional and nonconventional) and resonance effect. The effectiveness of each of these parameters on the stability of ligands is discussed. The prepared homoleptic complexes of [Ni(APBO)2] and [Cu(APBO)2] were characterized with IR, NMR, UV–Vis spectroscopic techniques. The X-ray crystallography of [Ni(APBO)2] demonstrated that the bidentate APBO binds to the metal center in trans fashion and the geometry around the nickel atom is square planar. The experimental studies on the complexes were accompanied computationally by the DFT and time-dependent DFT calculations.
Two new mixed-chelate dinuclear copper(II) complexes, [Cu2(tmen)2(TAP)]X2 where tmen = N,N,N′,N′-tetramethylethylenediamine, TAP = tetraacetylpropane, and X = or , were prepared and characterized by physicochemical and spectral (IR, UV–vis) data. The X-ray diffraction study of [{Cu(tmen)(CH3CN)}{Cu(tmen)(ClO4)}(TAP)](ClO4) demonstrated that coordination geometry around the copper centers is square pyramidal where axial position of one copper is occupied by a ClO4 − and the second copper with acetonitrile. However, in solution, the resulting complexes display affinity for axial ligation so that the apical ligands are driven out by solvent molecules. The solvatochromism of the complexes was investigated in various organic solvents and was compared with that of the corresponding mononuclear complex [Cu(tmen)(CH3-acac)]ClO4. A multi-parametric equation has been utilized to explain the solvent effect on the d–d transition of the complexes using SPSS/PC software. To explore the mechanism of the interaction between the solvent molecules and the complexes, different solvent parameters such as DN, AN, α, E T(30), π ∗, and β using stepwise multiple linear regression method were employed. In pyridine, the original color of the solution changed over time due to removal of tmen chelates and substitution by pyridine in two successive steps.
Two mixed-chelate copper(II) complexes that encompass N,N,N′,N′-tetramethylethylenediamine (tmen) and a β-ketoamine derivative were prepared. The elemental analysis, spectroscopic, conductance measurements and X-ray structural analysis of the newly prepared complexes are presented and discussed. The molar conductivity values in various solvents reveal the predominance of electrostatic interactions between the [Cu(tmen)(β-ketoamine)(H2O)]+ entity and the NO3− anion that counterbalances the positive charge. The resulting complexes, with a local symmetry of CuO2N3, attain nearly square-pyramidal structures and display an affinity for axial ligation. The tendency for the axial ligation is particularly fulfilled when suitable nucleophiles (solvents) with different donor abilities exist, leading to solvatochromism. The solute–solvent interactions are revealed by shifts in the ligand field absorption spectra, which are enhanced as the donor power of the solvent increases. A multi-parametric equation has been utilized to explain the solvent effect on the d–d transitions of the complexes using SPSS/PC software. To explore the mechanism of the interaction between the solvent molecules and the complexes, different solvent parameters such as DN (donor number), AN (acceptor number), α (hydrogen bonding ability), ET30 (Dimorth and Richardt’s), π∗ (polarity/polarizability parameter) and β (electron pair donating ability) using the multiple linear regression (MLR) method were employed. The results demonstrated that the donor power of the solvent plays the most important role in the solvatochromism of the compounds. A linear dependence of the ligand field absorption maximum on the solvent donor number is generally observed.
Molecular structure, vibration analysis, and natural bond orbital study of four derivatives of tetraketonate ligand were investigated in three different solvents using density functional theory B3LYP/6-31++G** method. The solution phase studies were carried out using an Onsager model. According to the obtained results the enol form of the ligands is more stable than keto form, even in solvents with high dielectric constant such as DMSO. This result is also confirmed experimentally using NMR studies for tetraacetylethane. Their stabilities are due to the presence of hydrogen bonding in the enol tautomers. A comparison among different possible enol forms of the substituted tetraketonate ligands demonstrated that three factors control the stability of the compounds as hydrogen bonding, steric hindrance, and charge distribution. The effectiveness of each of these factors on the stability of ligands depends on the nature of the substituent attached to the ligand.
Three new mixed-chelate Cu(II) complexes incorporating N,N,N′,N′-tetramethylenediamine (tmen) or N,N-dimethyl,N′-benzyl-ethylenediamine (dmben) as diamine chelate and a β-ketoaminato such as 4-amino-3-penten-2-onato (APO) or 3-amino-3-phenyl-2-buten-1-onato (APBO) with the general formula [Cu(β-ketoamine)(diamine)]ClO4 were prepared and characterized. Their solvatochromic properties were studied by visible spectroscopy. X-ray crystal analysis confirmed that copper (II) ion in [Cu(APO)(tmen)]ClO4 and[Cu(APBO)(tmen)]ClO4 is almost in a square planar environment. Structure of [Cu(APO)(dmben)]ClO4 was investigated by DFT calculation. The solvent-dependent visible spectroscopic absorption maxima, νmax, were analyzed using stepwise multiple linear regression (SMLR) method to find the best model explaining the observed positive solvatochromism. The analysis demonstrated that among different solvent parameters, donor number (DN) is a dominant factor responsible for the shift in the d–d absorption band of the complexes to the lower wavenumber with increasing its values. The importance of steric effect in the diamine ligand and more delocalization of pi-bands in the β-ketoamine on the spectral and SMLR measurements are discussed.
Four new symmetric mixed-chelate dinuclear complexes type [Cu2(L)2(TAE)]X2, where TAE = tetraacetylethane; L = N,N-dimethyl-N′-benzylethylenediamine (L1) or N,N′-dibenylethylenediamine (L2); X = ClO4 or BPh4 have been synthesized and characterized on the bases of elemental analysis, spectroscopic and conductance measurements. The X-ray crystal analysis of [Cu2(L1)2(TAE)](ClO4)2 demonstrated that the two copper(II) ions are not equivalent. The axial position of the first copper is occupied by a ClO4− ion with a square pyramidal geometry whereas; the second copper ion resides in an octahedral environment determined by two perchlorate anions. However, in solution, the perchlorate ions are driven out by solvent molecules leading to their solvatochromism. The solvatochromism of the complexes were investigated in various organic solvents and also were compared with those of the corresponding mononuclear complexes [Cu(L)(acac)]ClO4. Their solvatochromism were also investigated with different solvent parameters models using stepwise multiple linear regression (SMLR) method. The results suggested that the DN parameter of the solvent has the dominate contribution to the shift of the d–d absorption band of the complexes. The results demonstrated that the complexes with counter ions of BPh4 are more solvatochromic in very weak donor solvents owing to their disinclination in ion-pair formation.
The infrared and electronic absorption spectra of a series of new mixed-chelate copper(II) complexes that encompass N,N-dibenzylethylenediamine (diamine) and 3-substituted derivatives of acetylacetone (x-acacH) were studied. The IR and electronic absorption spectra and the molar conductivity of the newly prepared complexes are presented and discussed. The molar conductivity in dichloromethane reveals a predominance of electrostatic interactions between [Cu(diamine)(x-acac)]+ entity and perchlorate anions that counterbalance the positive charge. The resulting complexes with local symmetry of CuO2N2 attains a square-coplanar structure and exhibits the tendency for axial ligation, which is enhanced when an electron-attracting substituent is attached to the γ-position of acetylacetonate moiety. The tendency for axial ligation is particularly fulfilled when suitable nucleophiles (solvents) with deferent donor abilities exist, leading to solvatochromism. The solute–solvent interactions are revealed by shifts in the ligand field absorption spectra that are enhanced as the donor power of the solvent increases. Linear dependence of the ligand field absorption maximum on solvent donor number is generally observed.
A new mixed-chelate copper(II) complex with the general formula [Cu(Cl-acac)(diamine)]ClO4, where Cl-acac is 3-chloroacetylacetonate ion and diamine is N,N-dimethyl,N′-benzyl-1,2-diaminoethane, was prepared and characterized on the basis of elemental analysis, spectroscopic and conductance measurements. The mixed-chelate complex is soluble in various solvents and presents an interesting combination of solvato- and thermochromism. Its reversible chromotropism behavior is discussed in detail.
Two dinuclear complexes of [LCu(Cl)(μ-Cl)CuCl3][LCu(Cl)(μ-Cl)CuL(H2O)]Cl, 1 and [LCu(Br)(μ-Br)]2Br, 2, with a tridentate ligand of 3-((pyridin-2-ylmethyl)amino)propanamide, L, were prepared and characterized by physicochemical (elemental analyses, molar conductance measurements, thermogravimetry) and spectroscopic (IR, UV–vis) data. The crystal structures of compounds have been identified by single-crystal X-ray diffraction analyses and showed that the tridentate ligand L functions as an N2O-donor via the nitrogen atoms of the secondary amine and pyridyl moieties together with the oxygen atom of the amide group. The structural geometry about the copper(II) ions is a distorted square pyramid. The compounds are chromotropic and their reversible chromotropism was investigated by utilizing spectral analysis. The halochromism was due to structural change and followed by ionization of the coordinated water molecules and deprotonation of the secondary amine moiety. It was discovered that the solvatochromism of the compounds arisen from the structural change followed by the solvation of the vacant sites of the complexes. The compounds demonstrated ionochromism and sensitivity and selectivity towards CN− and N3− anions in the presence of other pseudo-halide anions.
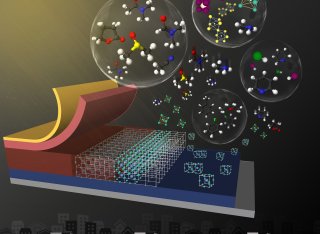
Organic–inorganic halide perovskite solar cells (PSCs) have shown a significant growth in power conversion efficiencies (PCEs) during last decade. Progress in device architecture and high-quality perovskite film fabrication has led to an incredible efficiency over 25% in close to a decade. Developments in solution-based thin film deposition techniques for perovskite layer preparation in PSCs provide low cost and ease of process for their manufacturing, making them a potential contender in future solar energy harvesting technologies. From small area single solar cells to large area perovskite solar modules, solvents play crucial roles in thin film quality and therefore, the device performance and stability. A comprehensive overview of solvent engineering toward achieving the highest qualities for perovskite light absorbing layers with various compositions and based on different fabrication processes is provided in this review. The mechanisms indicating the essential roles a solvent, or a solvent mixture can play to improve the crystallinity, uniformity, coverage and surface roughness of the perovskite films, are discussed. Finally, the role of solvent engineering in transferring from small area laboratory scale PSC fabrication to large area perovskite film deposition processes is explored.
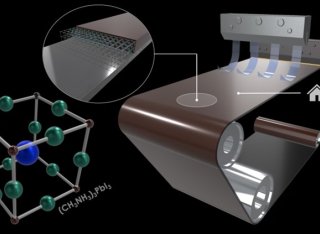
Halide perovskite materials have been extensively explored for their unique electrical, optical, magnetic, and catalytic properties. Most notably, solar cells based on perovskite thin films have improved their power conversion efficiency from 3.8% to over 25% during the last 12 years. However, it is still a challenge to develop a perovskite-based ink, suitable for upscaling the fabrication process of high-quality perovskite films with extreme purity, good crystallinity, and complete coverage over the deposition area. This is particularly important if the perovskite films are to be used for the scaled production of optoelectronic devices. Therefore, to make halide perovskites commercially available for various applications, it is vital to develop a reliable and highly robust deposition method, which can then be transferred to industry. Herein, the development of perovskite precursor inks suitable for use at low-temperature and vacuum-free solution-based deposition processes is reported. These inks can be further tailored according to the requirements of the deposition method, i.e., we propose their use with the industrially viable deposition technique called “slot-die coating”. Furthermore, a route for the preparation of low-cost and high-volume manufacturing of perovskite films on both rigid and flexible substrates is suggested in this paper. The presented approach is suitable for the fabrication of any functional layers of perovskites, that can be employed in various scaled applications, and it seeks the potential and the methodology for perovskite film deposition that is scalable to industrial standards.