Dr Simone Krings
Academic and research departments
Section of Bacteriology, Discipline: Microbes, Infection and Immunity, School of Biosciences.About
My research project
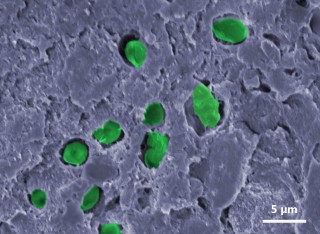
Encapsulating bacteria to create functional biocoatingsBiocoatings (or biocomposites) are “living paints” made from colloidal polymers (i.e. synthetic latex) which encapsulate functional, metabolically active bacteria. These biocoatings could be used for applications that do not require bioreactors and the unwanted biomass they produce. In addition, these “artificial biofilms” could facilitate the transport of functional microorganisms. In addition to the synthetic latex, our biocoatings’ formulation features halloysite nanotubes to increase the porosity and therefore to enhance gaseous exchanges and bacterial hydration. Our studies have been carried out using E. coli as a model organism, as well as cyanobacteria, the marine strain Synechococcus sp. PCC 7002 and the freshwater strain Synechocystis sp. PCC 6803. The microstructure of the biocoatings revealed bacterial encapsulation and a surrounding network of pores. Higher proportions of viable bacteria were found within halloysite biocoatings than in less porous coatings. Viability within our biocoatings was assessed after the film formation process using resazurin reduction assays or luminescence-based assays (CellTiter-Glo), as well as confocal light scanning microscopy (CLSM). The use of synergistically acting populations, genetic modifications or other bacterial species, such as acetogenic bacteria, could result in advances in bioremediation, wastewater treatment and sustainable energy production.
Supervisors
Biocoatings (or biocomposites) are “living paints” made from colloidal polymers (i.e. synthetic latex) which encapsulate functional, metabolically active bacteria. These biocoatings could be used for applications that do not require bioreactors and the unwanted biomass they produce. In addition, these “artificial biofilms” could facilitate the transport of functional microorganisms. In addition to the synthetic latex, our biocoatings’ formulation features halloysite nanotubes to increase the porosity and therefore to enhance gaseous exchanges and bacterial hydration. Our studies have been carried out using E. coli as a model organism, as well as cyanobacteria, the marine strain Synechococcus sp. PCC 7002 and the freshwater strain Synechocystis sp. PCC 6803. The microstructure of the biocoatings revealed bacterial encapsulation and a surrounding network of pores. Higher proportions of viable bacteria were found within halloysite biocoatings than in less porous coatings. Viability within our biocoatings was assessed after the film formation process using resazurin reduction assays or luminescence-based assays (CellTiter-Glo), as well as confocal light scanning microscopy (CLSM). The use of synergistically acting populations, genetic modifications or other bacterial species, such as acetogenic bacteria, could result in advances in bioremediation, wastewater treatment and sustainable energy production.
Publications
Biofilm bioreactors are attracting growing interest in the wastewater industry, as they allow higher cell densities and thus higher reaction rates compared to conventional bioreactors. However, some commonly used nitrifying bacteria, such as Nitrosomonas europaea, are slow-growing and need a prolonged period of time to develop a mature biofilm. Here, a biocoating or "living paint" is introduced, which is a synthetic biofilm made from a colloidal polymer (synthetic latex) binder encapsulating viable nitrifying bacteria at high density. Conventionally, the film formation of biocoatings is achieved by drying a bacteria/latex mixture. However, this fabrication is detrimental to the viability of the encapsulated bacteria because of the osmotic stress induced by desiccation. A nondesiccating film formation process is presented for biocoatings, which exploits two colloid science phenomena: coagulation and wet sintering. Desiccation-sensitive, nitrifying bacteria are employed in the biocoatings to convert NH4+ to NO2- and then NO3-. These biocoatings have a conversion rate (NO2- and NO3- production) of 3 mg N g(-1) d(-1) that is five times higher than in conventionally desiccated biocoatings. The reactivity continues over a period of 1 month. The processing method for these living paints is transformative for wastewater treatment and other applications using delicate, desiccation-sensitive microorganisms.
A biocoating confines non-growing, metabolically-active bacteria within a synthetic colloidal polymer (i.e. latex) film. Bacteria encapsulated inside biocoatings can perform useful functions, such as a biocatalyst in wastewater treatment. A biocoating needs to have high a permeability to allow a high rate of mass transfer for rehydration and the transport of both nutrients and metabolic products. It therefore requires an interconnected porous structure. Tuning the porosity architecture is a challenge. Here, we exploited rigid tubular nanoclays (halloysite) and non-toxic latex particles (with a relatively high glass transition temperature) as the colloidal “building blocks” to tailor the porosity inside biocoatings containing Escherichia coli bacteria as a model organism. Electron microscope images revealed inefficient packing of the rigid nanotubes and proved the existence of nanovoids along the halloysite/polymer interfaces. Single-cell observations using confocal laser scanning microscopy provided evidence for metabolic activity of the E. coli within the biocoatings through the expression of yellow fluorescent protein. A custom-built apparatus was used to measure the permeability of a fluorescein sodium salt in the biocoatings. Whereas there was no measurable permeability in a coating made from only latex particles, the permeability coefficient of the composite biocoatings increased with increasing halloysite content up to a value of 110-4 m h-1. The effects of this increase in permeability was demonstrated through a specially-developed resazurin reduction assay. Bacteria encapsulated in halloysite composite biocoatings had statistically significant higher metabolic activities in comparison to bacteria encapsulated in a non-optimized coating made from latex particles alone.
ABSTRACT Biocoatings, in which viable bacteria are immobilized within a waterborne polymer coating for a wide range of potential applications, have garnered greater interest in recent years. In bioreactors, biocoatings can be ready-to-use alternatives for carbon capture or biofuel production that could be reused multiple times. Here, we have immobilized cyanobacteria in mechanically hard biocoatings, which were deposited from polymer colloids in water (i.e ., latex). The biocoatings are formed upon heating to 37°C and fully dried before rehydrating. The viability and oxygen evolution of three cyanobacterial species within the biocoatings were compared. Synechococcus sp. PCC 7002 was non-viable inside the biocoatings immediately after drying, whereas Synechocystis sp. PCC 6803 survived the coating formation, as shown by an adenosine triphosphate (ATP) assay. Synechocystis sp. PCC 6803 consumed oxygen (by cell respiration) for up to 5 days, but was unable to perform photosynthesis, as indicated by a lack of oxygen evolution. However, Chroococcidiopsis cubana PCC 7433, a strain of desiccation-resistant extremophilic cyanobacteria, survived and performed photosynthesis and carbon capture within the biocoating, with specific rates of oxygen evolution up to 0.4 g of oxygen/g of biomass per day. Continuous measurements of dissolved oxygen were carried out over a month and showed no sign of decreasing activity. Extremophilic cyanobacteria are viable in a variety of environments, making them ideal candidates for use in biocoatings and other biotechnology. IMPORTANCE As water has become a precious resource, there is a growing need for less water-intensive use of microorganisms, while avoiding desiccation stress. Mechanically robust, ready-to-use biocoatings or “living paints” (a type of artificial biofilm consisting of a synthetic matrix containing functional bacteria) represent a novel way to address these issues. Here, we describe the revolutionary, first-ever use of an extremophilic cyanobacterium ( Chroococcidiopsis cubana PCC 7433) in biocoatings, which were able to produce high levels of oxygen and carbon capture for at least 1 month despite complete desiccation and subsequent rehydration. Beyond culturing viable bacteria with reduced water resources, this pioneering use of extremophiles in biocoatings could be further developed for a variety of applications, including carbon capture, wastewater treatment and biofuel production. As water has become a precious resource, there is a growing need for less water-intensive use of microorganisms, while avoiding desiccation stress. Mechanically robust, ready-to-use biocoatings or “living paints” (a type of artificial biofilm consisting of a synthetic matrix containing functional bacteria) represent a novel way to address these issues. Here, we describe the revolutionary, first-ever use of an extremophilic cyanobacterium ( Chroococcidiopsis cubana PCC 7433) in biocoatings, which were able to produce high levels of oxygen and carbon capture for at least 1 month despite complete desiccation and subsequent rehydration. Beyond culturing viable bacteria with reduced water resources, this pioneering use of extremophiles in biocoatings could be further developed for a variety of applications, including carbon capture, wastewater treatment and biofuel production.